Production
Hydrogen is not an energy source, but is an energy vector or carrier. This means that it has to be produced from one of the primary energy sources: fossil fuels, nuclear, solar, wind, biomass, hydro, geothermal and urban waste resources. All the energy we use, including hydrogen, must be produced from one of these three primary energy resources.
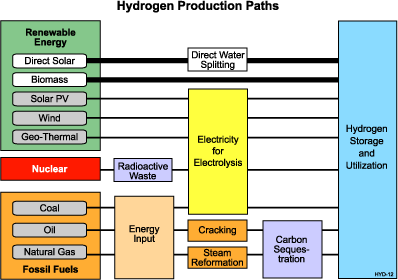
On earth, hydrogen is found combined with other elements. For example, in water, hydrogen is combined with oxygen. In fossil fuels, it is combined with carbon as in petroleum, natural gas or coal. The challenge is to separate hydrogen from other naturally occurring compounds in an efficient and economic manner. See the "Hydrogen Production Paths" chart below for unique ways to produce hydrogen from the three sources.
There are several methods for producing or extracting hydrogen. Steam reforming is a well-established technology that allows hydrogen production from hydrocarbons and water. Steam-methane reformation currently produces about 95 percent of the hydrogen used in the United States.
Another conventional technique is electrolysis, which applies electrical current to decompose water into hydrogen and oxygen molecules. The electricity for electrolysis can come from any of the three energy sources.
The cost of hydrogen production is an important issue. Hydrogen produced by steam reformation costs approximately three times the cost of natural gas per unit of energy produced. This means that if natural gas costs $6/million BTU, then hydrogen will be $18/million BTU. Also, producing hydrogen from electrolysis with electricity at 5 cents/kWh will cost $28/million BTU — slightly less than two times the cost of hydrogen from natural gas. Note that the cost of hydrogen production from electricity is a linear function of electricity costs, so electricity at 10 cents/kWh means that hydrogen will cost $56/million BTU.
On earth, hydrogen is found combined with other elements. For example, in water, hydrogen is combined with oxygen. In fossil fuels, it is combined with carbon as in petroleum, natural gas or coal. The challenge is to separate hydrogen from other naturally occurring compounds in an efficient and economic manner. See the "Hydrogen Production Paths" chart below for unique ways to produce hydrogen from the three sources.
There are several methods for producing or extracting hydrogen. Steam reforming is a well-established technology that allows hydrogen production from hydrocarbons and water. Steam-methane reformation currently produces about 95 percent of the hydrogen used in the United States.
Another conventional technique is electrolysis, which applies electrical current to decompose water into hydrogen and oxygen molecules. The electricity for electrolysis can come from any of the three energy sources.

The cost of hydrogen production is an important issue. Hydrogen produced by steam reformation costs approximately three times the cost of natural gas per unit of energy produced. This means that if natural gas costs $6/million BTU, then hydrogen will be $18/million BTU. Also, producing hydrogen from electrolysis with electricity at 5 cents/kWh will cost $28/million BTU — slightly less than two times the cost of hydrogen from natural gas. Note that the cost of hydrogen production from electricity is a linear function of electricity costs, so electricity at 10 cents/kWh means that hydrogen will cost $56/million BTU.
Energy Required (kWh/Nm3) |
|||||
|---|---|---|---|---|---|
Process |
Ideal |
Practical |
Status of Tech. |
Efficiency [%] |
Costs Relative to SMR |
| Steam methane reforming (SMR) | 0.78 |
2-2.5 |
mature |
70-80 |
1 |
| Methane/ NG pyrolysis | R&D to mature |
72-54 |
0.9 |
||
| H2S methane reforming | 1.5 |
- |
R&D |
50 |
<1 |
| Landfill gas dry reformation | R&D |
47-58 |
~1 |
||
| Partial oxidation of heavy oil | 0.94 |
4.9 |
mature |
70 |
1.8 |
| Naphtha reforming | mature |
||||
| Steam reforming of waste oil | R&D |
75 |
<1 |
||
| Coal gasification (TEXACO) | 1.01 |
8.6 |
mature |
60 |
1.4-2.6 |
| Partial oxidation of coal | mature |
55 |
|||
| Steam-iron process | R&D |
46 |
1.9 |
||
| Chloralkali electrolysis | mature |
by-product |
|||
| Grid electrolysis of water | 3.54 |
4.9 |
R&D |
27 |
3-10 |
| Solar & PV-electrolysis of water | R&D to mature |
10 |
>3 |
||
| High-temp. electrolysis of water | R&D |
48 |
2.2 |
||
| Thermochemical water splitting | early R&D |
35-45 |
6 |
||
| Biomass gasification | R&D |
45-50 |
2.0-2.4 |
||
| Photobiological | early R&D |
<1 |
|||
| Photolysis of water | early R&D |
<10 |
|||
| Photoelectrochemical decomp. of water | early R&D |
||||
| Photocatalytic decomp. of water | early R&D |
||||
